Joint research of ISU quantum chemists in collaboration with synthetic chemists from the Irkutsk Institute of Chemistry (Siberian Branch of the Russian Academy of Sciences) was published in the Journal of Organic Chemistry.
This Journal is listed in the first quartile of the international Web of Science database. Only articles of global significance are accepted for publication in this highly ranked journal.
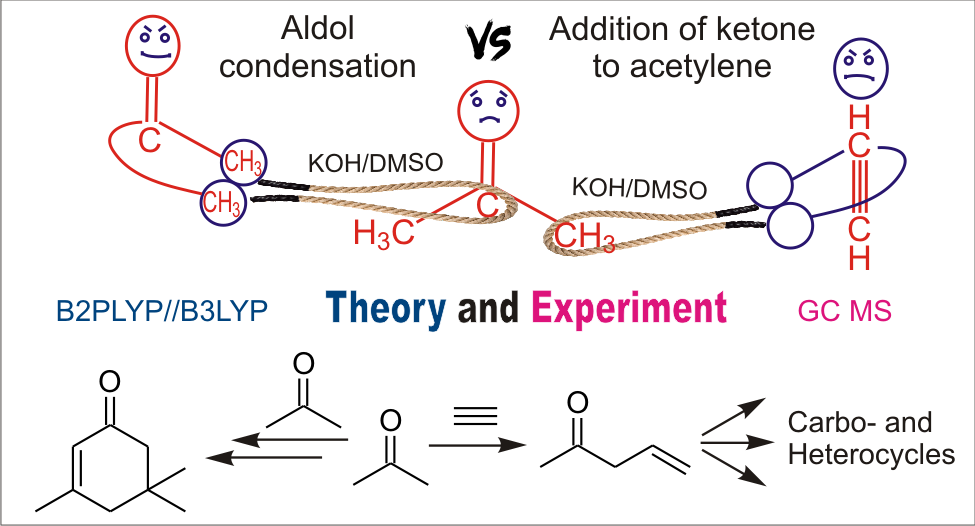
The mechanism of aldol condensation of ketones in KOH/DMSO superbasic media has been investigated using the B2PLYP(D2)/6-311+G**//B3LYP/6-31+G* quantum-chemical approach. It is found that the interaction of three ketone molecules resulting in the formation of the cyclohex-2-enone structure [isophorone or 3,5-dicyclohexyl-5-methylspiro(5.5)undec-2-en-1-one] is thermodynamically more favorable than the interaction of two, three, or four molecules of ketone, resulting in the formation of linear products of the condensation. The formation of the condensation products with the isophorone skeleton can significantly hinder the cascade reactions of ketones with acetylenes [to afford 6,8-dioxabicyclo(3.2.1)octanes or acylcyclopentenols] promoted by superbases. In particular, the kinetically more preferable reactions of autovinylation of 2-methyl-3-butyn-2-ol and autocondensation of acetone are the reasons why interaction of acetone with acetylene does not lead to the products of the cascade assemblies. The predominant formation of the products of these side reactions is confirmed experimentally.
Among the co-authors of the article are ISU scientists: Prof Nadezhda Vitkovskaya, (Doctor of Sciences (Chemistry), leading researcher; Prof Vladimir Orel (Doctor of Sciences (Chemistry), leading researcher; Dr Alexander Bobkov, PhD, senior researcher.